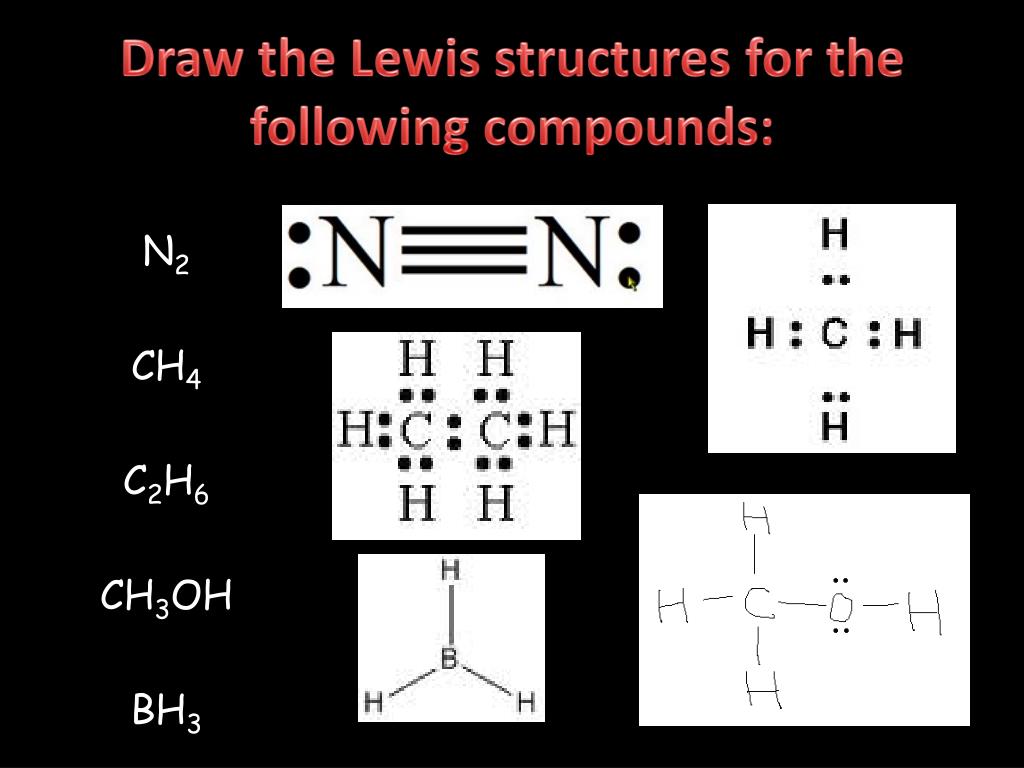
PROBLEM 4.2.7 4.2. 7. The arrangement of atoms in several biologically important molecules is given here. Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms. a. the amino acid serine: b. urea: c. pyruvic acid: d. uracil: e. carbonic acid:
Chemistry Partner: Lewis Structures of Some Common Compounds and Ions
In a similar sense, the two Lewis structures for the SO 2 molecule are in resonance. They mix to give a hybrid that is more than the sum of its components. The fact that SO 2 is a resonance hybrid of two Lewis structures is indicated by writing a double-headed arrow between these Lewis structures, as shown in the figure above.
Source Image: slideserve.com
Download Image
Contributors and Attributions Learning Objectives To draw Lewis Structures for molecules and polyatomic ions with one central atom. Introduction to Lewis structures A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.
Source Image: mdpi.com
Download Image
Electron Driven Reactions in Tetrafluoroethane: Positive and Negative Ion Formation | Journal of the American Society for Mass Spectrometry We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of

Source Image: study.com
Download Image
The Lewis Structures Of Four Compounds Are Given.
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of Additionally ionic compounds don’t exist as individual molecules (as their formula unit suggests) but as a repeating pattern of these formula units in a lattice. So you can use Lewis structures for ionic compounds too, but the bonding is different enough from covalent compounds that it’s simply not used to the same amount. Hope that helps.
Identifying Common Chemical Groups in a Lewis Structure | Chemistry | Study. com
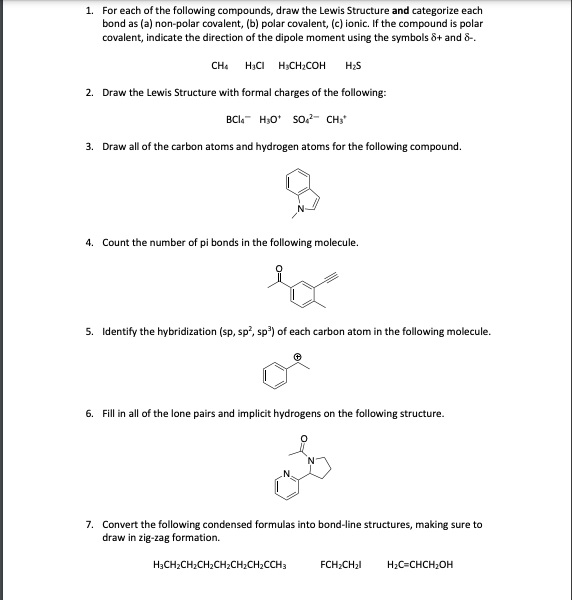
Chemistry Chemistry questions and answers The Lewis structures of four compounds are given. In the first molecule sufur is the central atom. There are two oxygen atoms bonded to the sulfur atom through double bonds. There is one lone pair of electrons on the sulfur atom and two lone pairs of electrons on each oxygen atom. Solved 1. For each of the following compounds, draw the | Chegg.com
Source Image: chegg.com
Download Image
Ethyne (Acetylene) – Structure, Properties, and Uses of C2H2 Chemistry Chemistry questions and answers The Lewis structures of four compounds are given. In the first molecule sufur is the central atom. There are two oxygen atoms bonded to the sulfur atom through double bonds. There is one lone pair of electrons on the sulfur atom and two lone pairs of electrons on each oxygen atom.

Source Image: byjus.com
Download Image
Chemistry Partner: Lewis Structures of Some Common Compounds and Ions PROBLEM 4.2.7 4.2. 7. The arrangement of atoms in several biologically important molecules is given here. Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms. a. the amino acid serine: b. urea: c. pyruvic acid: d. uracil: e. carbonic acid:

Source Image: chemistrypartner.blogspot.com
Download Image
Electron Driven Reactions in Tetrafluoroethane: Positive and Negative Ion Formation | Journal of the American Society for Mass Spectrometry Contributors and Attributions Learning Objectives To draw Lewis Structures for molecules and polyatomic ions with one central atom. Introduction to Lewis structures A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.

Source Image: pubs.acs.org
Download Image
9.5 Covalent Bonds and Lewis Structures – CHEM 1114 – Introduction to Chemistry Jan 23, 2023Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis

Source Image: pressbooks.bccampus.ca
Download Image
Chemistry | Free Full-Text | Chemistry, Synthesis, and Structure Activity Relationship of Anticancer Quinoxalines We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of
Source Image: mdpi.com
Download Image
7.3 Lewis Structures and Covalent Compounds – Introduction to Chemistry Additionally ionic compounds don’t exist as individual molecules (as their formula unit suggests) but as a repeating pattern of these formula units in a lattice. So you can use Lewis structures for ionic compounds too, but the bonding is different enough from covalent compounds that it’s simply not used to the same amount. Hope that helps.
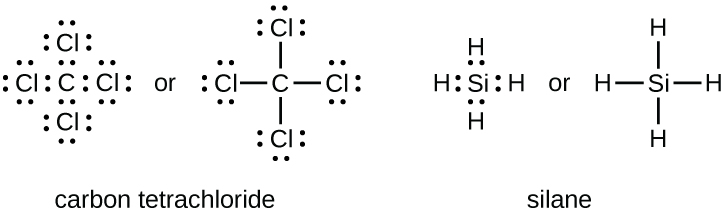
Source Image: openoregon.pressbooks.pub
Download Image
Ethyne (Acetylene) – Structure, Properties, and Uses of C2H2
7.3 Lewis Structures and Covalent Compounds – Introduction to Chemistry In a similar sense, the two Lewis structures for the SO 2 molecule are in resonance. They mix to give a hybrid that is more than the sum of its components. The fact that SO 2 is a resonance hybrid of two Lewis structures is indicated by writing a double-headed arrow between these Lewis structures, as shown in the figure above.
Electron Driven Reactions in Tetrafluoroethane: Positive and Negative Ion Formation | Journal of the American Society for Mass Spectrometry Chemistry | Free Full-Text | Chemistry, Synthesis, and Structure Activity Relationship of Anticancer Quinoxalines Jan 23, 2023Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis