Nov 28, 2022In order to complete the missing chemical formulas, you must first understand the conservation of matter, which states the count of individual atoms of each element should be the same on both the reactants and the products side. Understanding of molecular and empirical formulas can also assist in determining these missing formulas. Explanation:
Solved Fill in the missing chemical formulae in the tables | Chegg.com
VIDEO ANSWER: Give him two tables and we can fill in the missing formulas. The first one is eight, so four minus and as the acid. We have to come up with the formula Forest. The base is damaged. There is a basis for me when an acid donates a protons

Source Image: reddit.com
Download Image
Free Chemistry calculator – Calculate chemical reactions and chemical properties step-by-step

Source Image: nytimes.com
Download Image
Print Stock Illustration – Download Image Now – Weather, Chemistry, Chemical – iStock ISBN: 9781133109655 Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste Publisher: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste Chapter16: Acids And Bases Section: Chapter Questions Problem 56A See similar textbooks Related questions Question

Source Image: barnesandnoble.com
Download Image
Fill In The Missing Chemical Formulae In The Tables Below
ISBN: 9781133109655 Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste Publisher: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste Chapter16: Acids And Bases Section: Chapter Questions Problem 56A See similar textbooks Related questions Question These are constitutional isomers, compounds with the same molecular formula but different structures. Complete the following table by writing in the missing molecular formulas. What do the molecules have in common? What don’t the molecules have in common? Draw 3 constitutional isomers for the molecular formula C6H14 using skeletal
Kitchens of the Great Midwest: A Novel by J. Ryan Stradal, Paperback | Barnes & Noble®
Sep 13, 20231 Expert Answer Best Newest Oldest J.R. S. answered • 09/13/23 Tutor 5.0 (145) Ph.D. University Professor with 10+ years Tutoring Experience About this tutor › Recall that the conjugate acid and the base differ by a proton (H +) SO 42- conjugate acid = HSO 4- Br – conjugate acid = HBr OH – conjugate acid = HOH = H 2 O Upvote • 0 Downvote Fill in the missing items in the table below. Find Atomic number, Mass number number of protons – YouTube

Source Image: youtube.com
Download Image
Solved Fill in the missing chemical formulae in the tables | Chegg.com Sep 13, 20231 Expert Answer Best Newest Oldest J.R. S. answered • 09/13/23 Tutor 5.0 (145) Ph.D. University Professor with 10+ years Tutoring Experience About this tutor › Recall that the conjugate acid and the base differ by a proton (H +) SO 42- conjugate acid = HSO 4- Br – conjugate acid = HBr OH – conjugate acid = HOH = H 2 O Upvote • 0 Downvote
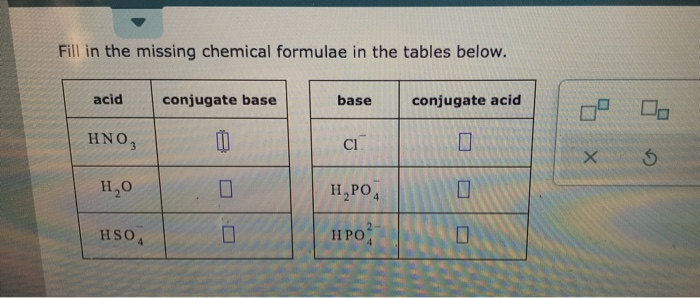
Source Image: chegg.com
Download Image
Solved Fill in the missing chemical formulae in the tables | Chegg.com Nov 28, 2022In order to complete the missing chemical formulas, you must first understand the conservation of matter, which states the count of individual atoms of each element should be the same on both the reactants and the products side. Understanding of molecular and empirical formulas can also assist in determining these missing formulas. Explanation:
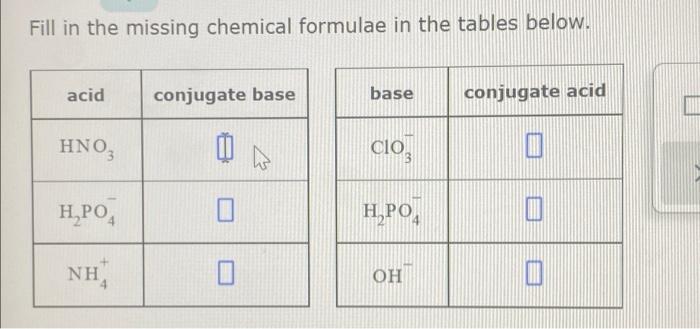
Source Image: chegg.com
Download Image
Print Stock Illustration – Download Image Now – Weather, Chemistry, Chemical – iStock Free Chemistry calculator – Calculate chemical reactions and chemical properties step-by-step

Source Image: istockphoto.com
Download Image
SOLVED: Fill in the missing chemical formulae in the tables below: acid conjugate base base conjugate acid HSO OH H,O H,o NH NH; HCIO , clo] ClO Oct 30, 2022ANSWERED STEP-BY-STEP Fill in the missing chemical formulae in the tables below. \begin tabular c \hline acid & conjugate base \\ \hline \ ( \mathrm NH_ 4^ + \) & \ ( \square \) \\ \hline \ ( \mathrm H_ 2 \mathrm SO_ 4 \) & \ ( \square \) \\ \hline \ ( \mathrm H_ 2 \mathrm O \) & \ ( \square \) \\ \hline
![SOLVED: Fill in the missing chemical formulae in the tables below: acid conjugate base base conjugate acid HSO OH H,O H,o NH NH; HCIO , clo] ClO](https://cdn.numerade.com/project-universal/previews/e3ee724c-db31-4d3b-8de4-9385b730b575.gif)
Source Image: numerade.com
Download Image
Sustainability | Free Full-Text | Attitude, Self-Control, and Prosocial Norm to Predict Intention to Use Social Media Responsibly: From Scale to Model Fit towards a Modified Theory of Planned Behavior ISBN: 9781133109655 Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste Publisher: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste Chapter16: Acids And Bases Section: Chapter Questions Problem 56A See similar textbooks Related questions Question
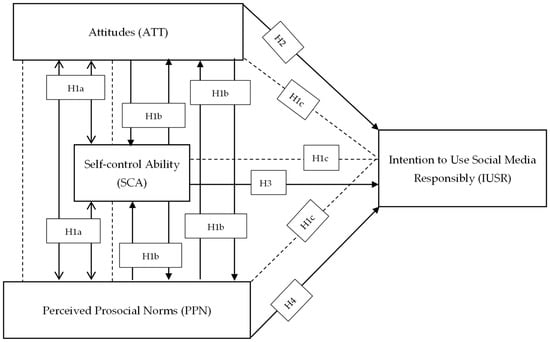
Source Image: mdpi.com
Download Image
Frontiers | Abundance of Environmental Data vs. Low Public Interest in Climate and Ocean Issues. Where Is the Missing Link? These are constitutional isomers, compounds with the same molecular formula but different structures. Complete the following table by writing in the missing molecular formulas. What do the molecules have in common? What don’t the molecules have in common? Draw 3 constitutional isomers for the molecular formula C6H14 using skeletal
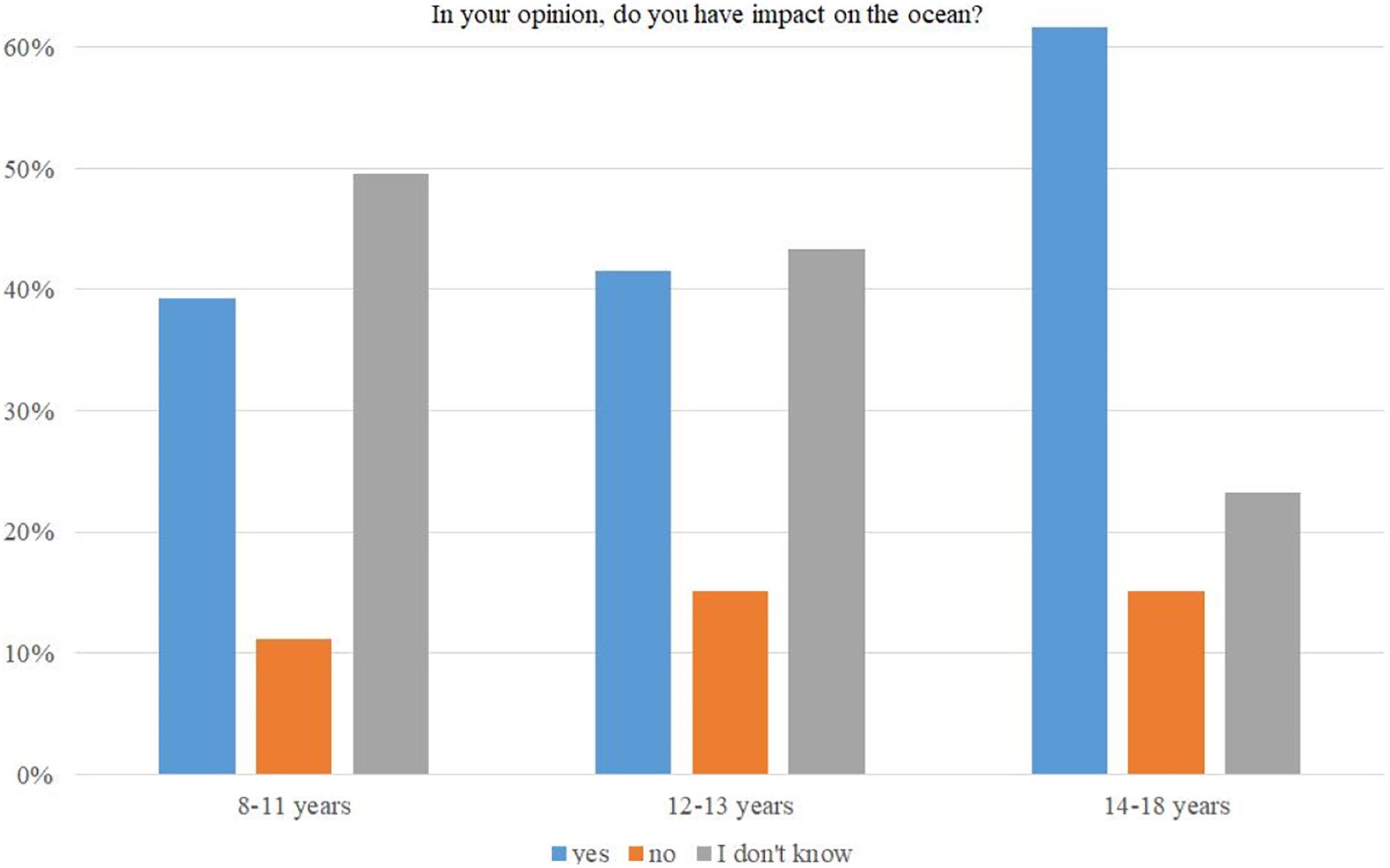
Source Image: frontiersin.org
Download Image
Solved Fill in the missing chemical formulae in the tables | Chegg.com
Frontiers | Abundance of Environmental Data vs. Low Public Interest in Climate and Ocean Issues. Where Is the Missing Link? VIDEO ANSWER: Give him two tables and we can fill in the missing formulas. The first one is eight, so four minus and as the acid. We have to come up with the formula Forest. The base is damaged. There is a basis for me when an acid donates a protons
Print Stock Illustration – Download Image Now – Weather, Chemistry, Chemical – iStock Sustainability | Free Full-Text | Attitude, Self-Control, and Prosocial Norm to Predict Intention to Use Social Media Responsibly: From Scale to Model Fit towards a Modified Theory of Planned Behavior Oct 30, 2022ANSWERED STEP-BY-STEP Fill in the missing chemical formulae in the tables below. \begin tabular \hline acid & conjugate base \\ \hline \ ( \mathrm NH_ 4^ + \) & \ ( \square \) \\ \hline \ ( \mathrm H_ 2 \mathrm SO_ 4 \) & \ ( \square \) \\ \hline \ ( \mathrm H_ 2 \mathrm O \) & \ ( \square \) \\ \hline